It is mainly used in the production of synthetic cryolite (Na 3 AlF 6), aluminium fluoride (AlF 3), motor gasoline alkylates and chlorofluorocarbons (CFCs). Where there was not sufficient information to establish EARs and RDAs, an estimate designated Adequate Intake (AI) was used instead. Solution for Calculate the solubility of barium fluoride (BaF2) in each of the following solutions. [34] Beryllium fluoride and aluminium fluoride are also used as phosphatase inhibitors, since these compounds are structural mimics of the phosphate group and can act as analogues of the transition state of the reaction. Thus, the option C is incorrect. [59], Around one-third of the human population drinks water from groundwater resources. (download: www.eawag.ch/en/research/humanwelfare/drinkingwater/wrq/geogenic-contamination-handbook/), Centers for Disease Control and Prevention, Fluorine-19 nuclear magnetic resonance spectroscopy, "Fluorides – PubChem Public Chemical Database", "Public Health Statement for Fluorides, Hydrogen Fluoride, and Fluorine", "Ambient Water Quality Criteria for Fluoride", "Fluoride in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality", "Fluoride in Drinking Water: A Review of Fluoridation and Regulation Issues", "Black tea--helpful or harmful? Although there is information to set Adequate Intake, fluoride does not have a Daily Value and is not required to be shown on food labels. The AI for men is 4.0 mg/day. Fasting dramatically increases the rate of fluoride absorption to near 100%, from a 60% to 80% when taken with food. Which of the following is correct explanation for the above given statement ? Haryana Govt. Values ranging from 0.46 to 3.6–5.4 mg/day have been reported in several studies (IPCS, 1984). to Euclids Geometry, Areas It is used in aluminium smelting. [33] Fluoride mimics the nucleophilic hydroxide ion in these enzymes' active sites.
(b) Statement -1 is True, Statement -2 is True, Statement -2 is NOT a correct explanation for Statement -1. [58], In areas that have naturally occurring high levels of fluoride in groundwater which is used for drinking water, both dental and skeletal fluorosis can be prevalent and severe. The difluorides of the transition metals often adopt the rutile structure whereas the dichlorides have cadmium chloride structures. Naked fluoride is a strong Lewis base,[17] and a powerful nucleophile. Problems with the usual explanations. p-Block Elements - I Vidyamandir Classes Exception : The solubility of alkali metal fluorides, hydroxides and carbonates increases rapidly down the group. For older children and adults, who are no longer at risk for dental fluorosis, the upper limit of fluoride is set at 10 mg/day regardless of weight. [44], According to the U.S. Department of Agriculture, the Dietary Reference Intakes, which is the "highest level of daily nutrient intake that is likely to pose no risk of adverse health effects" specify 10 mg/day for most people, corresponding to 10 L of fluoridated water with no risk. DOI: 10.1063/1.2741386. Thus, the option B is incorrect. Relative unsolvated fluoride, which does exist in aprotic solvents, is called "naked". [63] Gloves made of nitrile rubber are worn when handling fluoride compounds.
Statement-2: The loss and gain of electron(s) can be used in explaining the reducing and oxidising behaviour of the element respectively. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin. Fluorine, in the form of fluoride, is considered to be a micronutrient for human health, necessary to prevent dental cavities, and to promote healthy bone growth. [18] Cobaltocenium fluoride is another example.
(C ) Statement -1 is True, Statement -2 is False. Perhaps the simplest case involving limited (<0.1 molar) solubility is lithium fluoride, whose solubility is only about one part in 750 by mass at 25°C (about 0.05 molar). Try it now. Fluoride salts typically have distinctive bitter tastes, and are odorless. [39][41], The upper limit of fluoride intake from all sources (fluoridated water, food, beverages, fluoride dental products and dietary fluoride supplements) is set at 0.10 mg/kg/day for infants, toddlers, and children through to 8 years old. This behaviour of lithium is attributed to its small size and very high hydration energy. Jharkhand Board: class 10 & 12 board exams will be held from 9th to 26th March 2021. However, they may require a prescription. Ksp at 25 °C for BaF2 = 1.8 x 10-7. bhi. A Review of Sodium Fluoride Solubility in Water. Formerly, it was mined, but now it is derived from hydrogen fluoride. [26] Originally, sodium fluoride was used to fluoridate water; hexafluorosilicic acid (H2SiF6) and its salt sodium hexafluorosilicate (Na2SiF6) are more commonly used additives, especially in the United States. Sulfates are soluble except those of calcium, strontium and barium 6. The systematic name fluoride, the valid IUPAC name, is determined according to the additive nomenclature. Punjab Schools Reopen for Class 5 to 11, Issues Fresh Covid-19 SOP. AIs are typically matched to actual average consumption, with the assumption that there appears to be a need, and that need is met by what people consume. The low concentration of fluoride reflects the insolubility of the alkaline earth fluorides, e.g., CaF2. Of this, about 10%, approximately three hundred million people, obtains water from groundwater resources that are heavily contaminated with arsenic or fluoride. Join the 2 Crores+ Student community now! Which of the following correct statement for the given acids? Know Himachal board syllabus, admit card & result. This explains why NaCl is soluble because Na is a group 1 metal and Cl is chloride. Fluoride is naturally present at low concentration in most fresh and saltwater sources, as well as in rainwater, particularly in urban areas. For air [19] However, they all lack structural characterization in aprotic solvents. Pan HB(1), Darvell BW. School Students from Class 8 to 12 will Get Free Tablets. J. M. Canich, G. L. Gard. [56] Fluoride was known to enhance the measurement of bone mineral density at the lumbar spine, but it was not effective for vertebral fractures and provoked more non vertebral fractures. Sources of true F− anions are rare because the highly basic fluoride anion abstracts protons from many, even adventitious, sources. Solutions of inorganic fluorides in water contain F− and bifluoride HF−2. Page 1 of 1. Johnson, A. Bretzler (Eds. Solubility of groups `1` and `2` fluorides increases down the group'. Also compounds containing chloride, bromide, and iodide are almost always soluble.
(e) Statement -1 and Statement -2 both are False. In CaCl2, each Ca2+ ion is surrounded by six Cl− centers. Explained", "Systematic review of water fluoridation", "Ten Great Public Health Achievements in the 20th Century", "Fluoride toothpastes of different concentrations for preventing dental caries", "Properties of a phosphoprotein phosphatase from bovine heart with activity on glycogen synthase, phosphorylase, and histone", "Crystal structures of a purple acid phosphatase, representing different steps of this enzyme's catalytic cycle", "BeF(3)(-) acts as a phosphate analog in proteins phosphorylated on aspartate: structure of a BeF(3)(-) complex with phosphoserine phosphatase", "Room-temperature cycling of metal fluoride electrodes: Liquid electrolytes for high-energy fluoride ion cells", "Fluoride-ion battery runs at room temperature", "Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies", "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. All tea leaves contain fluoride; however, mature leaves contain as much as 10 to 20 times the fluoride levels of young leaves from the same plant.[13][14][15]. The hydrides of Group 1 metals are white crystalline solids which contain the metal ions and hydride ions, H-. It is therefore a weak base, and tends to remain as the fluoride ion rather than generating a substantial amount of hydrogen fluoride. 3: TOXICITY AND SOLUBILITY OF DIFFERENT FLUORIDES [1] Professor Kaj Roholm's three categories of inorganic fluorine compounds. Himachal Board Exam Dates 2021 for Class 12, 10 Announced, Datesheet Soon. Fluorine is estimated to be the 13th-most abundant element in the earth's crust and is widely dispersed in nature, entirely in the form of fluorides. Statement-1: Metallic character of first group metals increases down the group with the decreasing ionisation energy. Jharkhand Board: Class 10 and 12 Exams Starts from 9th March, 2021. The compounds in this group are water-soluble fluoride salts which can react with trace amounts of water to form the dangerous acid hydrogen fluoride, or hydrofluoric acid. 7. [25] For this reason, it is used in toothpaste and water fluoridation. [35][36], A large team of researchers, including Simon C. Jones of California Institute of Technology and Christopher J. Brooks of the Honda Research Institute, have come up with a liquid electrolyte that shuttles fluoride ions to and fro and demonstrated its use in a room-temperature, rechargeable FIB (Science 2018, DOI: 10.1126/science.aat7070). [55] In the lower doses used for water fluoridation, the only clear adverse effect is dental fluorosis, which can alter the appearance of children's teeth during tooth development; this is mostly mild and is unlikely to represent any real effect on aesthetic appearance or on public health. In periodic table, melting point/boiling point increases down the group in which of the following group ? In other locations the level of fluoride is very low, sometimes leading to, This page was last edited on 29 November 2020, at 01:53. [23] In areas where water is fluoridated this can be expected to be a significant source of fluoride, however fluoride is also naturally present in virtually all foods and beverages at a wide range of concentrations. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Circumstances of Poisoning. [57], A popular urban myth claims that the Nazis used fluoride in concentration camps, but there is no historical evidence to prove this claim. Fluoride is also used non-systematically, to describe compounds which release fluoride upon dissolving.
Statement-I: p-chloro phenol is more acidic than p-flouro phenol. There is little data for beryllium carbonate, but as it reacts with water, the trend is obscured. Punjab schools reopen for class 5 to 11, issues fresh Covid-19 SOP. [32], Fluoride salts are commonly used in biological assay processing to inhibit the activity of phosphatases, such as serine/threonine phosphatases. [54], In the higher doses used to treat osteoporosis, sodium fluoride can cause pain in the legs and incomplete stress fractures when the doses are too high; it also irritates the stomach, sometimes so severely as to cause ulcers. [4] The soft, colorful mineral is found worldwide. There are some cations whose Group I/ammonium salts are not soluble in water. As for safety, the IOM sets tolerable upper intake levels (ULs) for vitamins and minerals when evidence is sufficient. [29][30] In some countries where large, centralized water systems are uncommon, fluoride is delivered to the populace by fluoridating table salt. Related to Circles, Introduction Announcements Applying to uni? BeF2 is very soluble in water due to the high hydration energy of the small Be+2ion. Question From class 11 Chapter PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY, FAQs on Periodic Classification Of Elements And General Inorganic Chemistry, Periodic Law And Intro To Periods And Groups, TRENDS IN PHYSICAL PROPERTIES - ATOMIC RADIUS, Paiye sabhi sawalon ka Video solution sirf photo khinch kar. Statement-1: All noble gases are considered as unreactive. [48][49][50] A case of a fatal poisoning of an adult with 4 grams of sodium fluoride is documented,[51] and a dose of 120 g sodium fluoride has been survived. These AIs are comparable to the U.S. school students from class 8 to 12 will get free tablets to study amid COVID-19 pandemic. #1 Report Thread starter 3 years ago #1 Why is the trend in melting points of group 1 fluorides… Exception: Group I fluoride salts are soluble.
(a) Statement -1 is True, Statement -2 is True , Statement -2 is a correct explanation for Statement -1. Caesium fluoride can be used in organic synthesis as a source of the fluoride anion. Solubility of alkali metal fluorides in water __________ down the group. Fluoride therapy for osteoporosis", "A systematic review of the efficacy and safety of fluoridation", "Truth about fluoride doesn't include Nazi myth", "Groundwater arsenic contamination throughout China", U.S. government site for checking status of local water fluoridation, https://en.wikipedia.org/w/index.php?title=Fluoride&oldid=991244655, Biology and pharmacology of chemical elements, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Articles with unsourced statements from September 2017, Creative Commons Attribution-ShareAlike License. For comparison, chloride concentration in seawater is about 19 g/L. Therefore whatever little solubility these fluorides have that increase down the group. For infants and young children the values are smaller, ranging from 0.7 mg/d for infants to 2.2 mg/d. Upon treatment with a standard acid, fluoride salts convert to hydrogen fluoride and metal salts. Post-Platinum Metals: Synthesis of High-Valent Fluorides, Oxide Fluorides … The hazards of solutions of fluoride salts depend on the concentration. For men the value is 3.4 mg/day. [39], The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values, with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. Many minerals are known, but of paramount commercial importance is fluorite (CaF2), which is roughly 49% fluoride by mass. Oxidation of fluoride gives fluorine. The identity of the solvent can have a dramatic effect on the equilibrium shifting it to the right-hand side, greatly increasing the rate of decomposition. The other fluorides (MgF2, CaF2, SrF2 and BaF2) are almost insoluble in water. Fluoride can act as a base. [20] The sterically demanding imidazolium cation stabilizes the discrete anions and protects them from polymerization.[21][22]. Rep:? AI and UL defined the same as in United States. [42], Daily intakes of fluoride can vary significantly according to the various sources of exposure. [41], For U.S. food and dietary supplement labeling purposes the amount of a vitamin or mineral in a serving is expressed as a percent of Daily Value (%DV). Collectively the EARs, RDAs, AIs and ULs are referred to as Dietary Reference Intakes (DRIs). They have exactly the same crystal structure as sodium chloride - that's why they are called saline or … Chemosphere 2020, 239, 124662. The ease of formation of alkali metal halides increases from Li to Cs 17. ? For children ages 1–17 years the AIs increase with age from 0.6 to 3.2 mg/day. (C) The solubility of hydroxides, fluorides and oxalates increases from calcium to barium. HF is miscible with water (will dissolve in any proportion), while the other hydrogen halides have large solubility gaps with water. [43] The maximum safe daily consumption of fluoride is 10 mg/day for an adult (U.S.) or 7 mg/day (European Union). of Derivatives, Application know JEE advanced 2021 exam latest update, important dates, eligibility criterion & application process! In late 2016 imidazolium fluoride was synthesized that is the closest approximation of a thermodynamically stable and structurally characterized example of a "naked" fluoride source in an aprotic solvent (acetonitrile). In the case of fluoride the UL is 10 mg/day. In the presence of strong acids, fluoride salts release hydrogen fluoride, which is corrosive, especially toward glass.[4]. Fluoridation of water has its critics (see Water fluoridation controversy). and Inverse Proportions, Areas [52] For sodium fluorosilicate (Na2SiF6), the median lethal dose (LD50) orally in rats is 0.125 g/kg, corresponding to 12.5 g for a 100 kg adult. Find your group chat here >> start new discussion reply. The AI for children ages 1–18 increases from 0.7 to 3.0 mg/day. Solubility of calcium fluoride and fluorapatite by solid titration. The main uses of fluoride, in terms of volume, are in the production of cryolite, Na3AlF6. DOI: 10.1021/acs.jced.7b00089. Know Steps to download Jharkhand board date sheet, syllabus, sample papers & more. The fluorine compounds decompose into products including fluoride ions. Fluoride (/ˈflʊəraɪd, ˈflɔːr-/)[3] is an inorganic, monatomic anion with the chemical formula F− (also written [F]−), whose salts are typically white or colorless. Since on descending the group lattice energy decreases more rapidly than the hydration energy. They provide a ready source of fluoride … [40] The EFSA reviewed safety evidence and set an adult UL at 7.0 mg/day (lower for children). At physiological pHs, hydrogen fluoride is usually fully ionised to fluoride. At much higher doses and frequent exposure, fluoride causes health complications and can be toxic. [46] Water and food sources of fluoride include community water fluoridation, seafood, tea, and gelatin. The Group 1 hydrides. In aqueous solution, fluoride has a pKb value of 10.8.
Statement -1: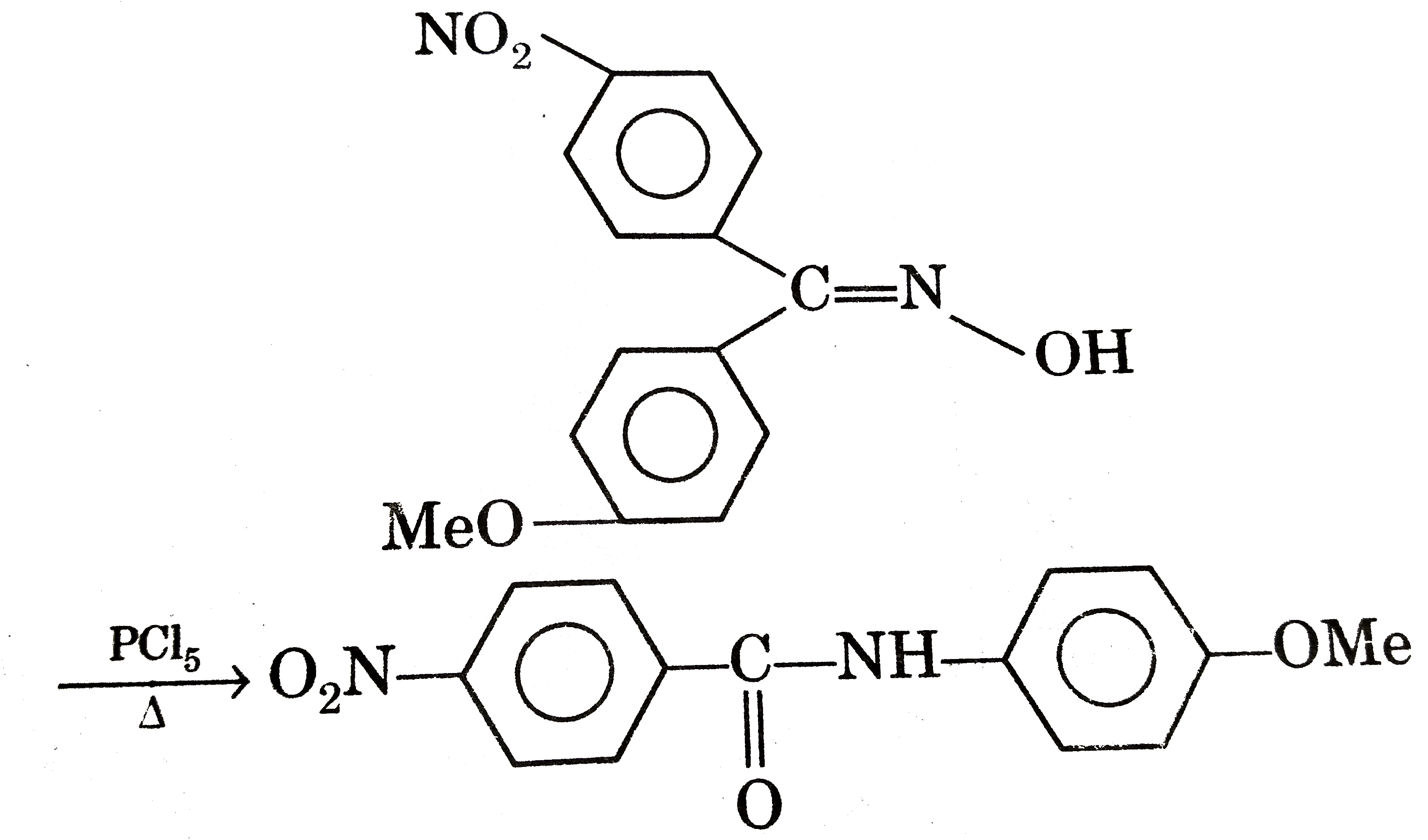
Statement -2: Migratory aptitude of p-methoxyphenyl group is greater than migratory aptitude p-nitrophenyl of group during carbrocation rearrangements. [4], Fluoride-containing compounds, such as sodium fluoride or sodium monofluorophosphate are used in topical and systemic fluoride therapy for preventing tooth decay. Mined fluorite (CaF2) is a commodity chemical used in steel-making. The reason is L. E. is proportional to 1/(r r ) and thus the lattice energy will vary most when r is small (as in fluorides) and least when r is longer (with I ).Thus the change in lattice enthalpy Hydrofluoric acid and its anhydrous form, hydrogen fluoride, is also used in the production of fluorocarbons. Just learn that Group 1 compounds tend to be more soluble than their Group 2 equivalents. For women ages 18 and older the AI is set at 2.9 mg/day (includes pregnancy and lactation). For compounds containing more than one fluoride per cation, the structures often deviate from those of the chlorides, as illustrated by the main fluoride mineral fluorite (CaF2) where the Ca2+ ions are surrounded by eight F− centers. Solubility of the carbonates. [47], Soluble fluoride salts, of which sodium fluoride is the most common, are toxic, and have resulted in both accidental and self-inflicted deaths from acute poisoning. 2007 Sep;52(9):861-8. C.A. Fluoride is the most bioavailable form of fluorine, and as such, tea is potentially a vehicle for fluoride dosing. Carl Francis Z. Lacson, Ming-Chun Lu, Yao-Hui Huang. "Solubility of alkali metal fluorices increases down the group " Select correct explanation for given statement, Statement: I : The solubility of aldehydes and ketones in water decreases with increase of size of the alkyl group
Statement - :II: Alkyl groups are eletron releasing groups. The major known risk of fluoride deficiency appears to be an increased risk of bacteria-caused tooth cavities. Fluorides include compounds that contain ionic fluoride and those in which fluoride does not dissociate. Journal of Chemical & Engineering Data 2017, 62 (6) , 1743-1748. Magnesium carbonate (the most soluble Group 2 carbonate) has a solubility of about 0.02 g per 100 g of water at room temperature. and Differentiability. Statement -1 Solubility of alcohols decreases with increasing molecular weight
Statement -2 : Increases in hydrophobic group decreases proportion of hydrogen bonding . Caesium also has the highest electropositivity of all non-radioactive elements and fluorine has the highest electronegativity of all elements. Many minerals are known, but of paramount commercial importance is fluorite (CaF2), which is roughly 49% fluoride by mass. Algebraic The current AI for women 19 years and older is 3.0 mg/day (includes pregnancy and lactation). In terms of its reactivity, fluoride differs significantly from chloride and other halides, and is more strongly solvated in protic solvents due to its smaller radius/charge ratio. Its closest chemical relative is hydroxide, since both have similar geometries. As we move down the group ,the solubility of alkali metal fluorides increases regularly as we move from LiF to CsF since the decrease in lattice enthalpy more than compensate the decrease in hydration enthalpy. Fluoride network and circular economy as potential model for sustainable development-A review. However, the name fluoride is also used in compositional IUPAC nomenclature which does not take the nature of bonding involved into account. Most fluoride salts dissolve to give the bifluoride (HF2−) anion. Hydroxide salts Rule I: Hydroxide salts of Group II elements are slightly soluble, including Ca(OH) 2, Sr(OH) 2, and Ba(OH) 2. Apne doubts clear karein ab Whatsapp (8 400 400 400) par Give the correct order of initials T or F for following statements Use T if statements is true and F if it is false. Author information: (1)Dental Materials Science, Faculty of Dentistry, The University of Hong Kong, Hong Kong, China. Some quaternary ammonium salts of naked fluoride include tetramethylammonium fluoride and tetrabutylammonium fluoride. Low solubility of LiF (0.27 g/100 g H2O ) is due to its high lattice energy ( - 1005 KJmol-1) whereas the low solubility of CsI (44g/100g H2O ) is due to smaller hydration energy of the two ions (-670 KJ/mol) . FR page 33982", "Food Composition Databases: Food Search: Fluoride", "Dietary Reference Intakes: EAR, RDA, AI, Acceptable Macronutrient Distribution Ranges, and UL", "Prevention and management of osteoporosis: consensus statements from the Scientific Advisory Board of the Osteoporosis Society of Canada. Saline (salt-like) hydrides.
Statement-II: Amongst elements of Group 15 the property which increases down the group is. [24] Approximately, 50% of absorbed fluoride is excreted renally with a twenty-four-hour period. You should not need it for UK A level purposes for Group 1. Fluorine is estimated to be the 13th-most abundant element in the earth's crust and is widely dispersed in nature, entirely in the form of fluorides.
(d) Statement -1 is False, Statement -2 is True. [24] Per a 2013 study, it was found that consumption of one litre of tea a day, can potentially supply the daily recommended intake of 4 mg per day. [7] Groundwater (well water) concentrations vary even more, depending on the presence of local fluoride-containing minerals. Hydrogen fluoride and water also form several compounds in the solid state, most notably a 1:1 compound that does not melt until −40 °C (−40 °F), which is 44 degrees Celsius (79 degrees Fahrenheit) above the melting point of pure HF. The solubility of the most of alkali metal halides except those of fluorides decreases on descending the group since the decrease in hydration energy is more than the corresponding decrease in the lattice … Fluoride salts and hydrofluoric acid are the main fluorides of industrial value. Surface water such as rivers or lakes generally contains between 0.01–0.3 ppm. It should be noted that Prof. Roholm is the author of the first and most comprehensive monograph on fluorine toxicity.
Statement-2: Down the group, melting and boiling point increases. A saturated solution has a concentration of about 1.3 g per 100 g of water at 20°C. The carbonates become less soluble down the group. Problems with the data. Hydrogen fluoride is itself an example of a non-systematic name of this nature. Numbers and Quadratic Equations, Introduction It is also used in etching semiconductor devices, cleaning and etching glass, cleaning brick and aluminium and tanning … Magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. Exceptions: Silver salts AgNO 3 and Ag(C 2 H 3 O 2) are soluble. of Parallelograms and Triangles, Introduction A review of the evidence", "Fluoride Free Toothpaste – Fluoride (Finally!) For example, natural levels of under 0.05 mg/L have been detected in parts of Canada but up to 8 mg/L in parts of China; in general levels rarely exceed 10 mg/litre[8], Fluoride can be present in rain, with its concentration increasing significantly upon exposure to volcanic activity or atmospheric pollution derived from burning fossil fuels or other sorts of industry. However, it is also a trivial name, and the preferred IUPAC name for fluorane. Because of their high basicity, many so-called naked fluoride sources are in fact bifluoride salts. Bihar board class 10 admit card 2021 latest update. [16] Few inorganic fluorides are soluble in water without undergoing significant hydrolysis. JEE advanced 2021 exam date on 03 July, 75% criterion removed. Mouth rinses and mouth washes typically contain about 0.05% sodium fluoride (225 ppm fluoride). Go to first unread Skip to page: HelloGoodbye Badges: 21. [11][12], All vegetation contains some fluoride, which is absorbed from soil and water. For example, sulfur hexafluoride and carbon tetrafluoride are not sources of fluoride ions under ordinary conditions. For the method of action for cavity prevention, see Fluoride therapy. A 0.76 g MFP is equivalent to 0.1 g fluoride. So sulphates and carbonates become less soluble as you go down the Group; hydroxides become more soluble. Sodium fluoride and sodium chloride adopt the same structure. 16. Concentrations in fresh water vary more significantly. In the situation of CaF2, Calcium is a group 2 metal and is most of the time not soluble and fluoride is not part of the soluble halogen group. Each question has 5 choices (a) ,(b) ,(c ) (d) and (e ) out of which ONLY ONE is correct. Expressions and Identities, Direct Epub 2007 Apr 23. [23] The tea plant (Camellia sinensis L.) is a known accumulator of fluorine compounds, released upon forming infusions such as the common beverage. Fasting can increase this to 150%. In Lassaigne's test, the organic compound is first fused with Sodium Metal instead of any other Group 1 Metal, Why is this so? Salts containing fluoride are numerous and adopt myriad structures. [60] These trace elements derive mainly from minerals. Organic and inorganic anions are produced from fluoride, including: This article is about the fluoride ion. Unfortunately, the enthalpy of solution values for the Group 1 chlorides as calculated above don't agree with the values given in the same Data Book: [62], Concentrated fluoride solutions are corrosive. Haryana Govt. Journal of Physical and Chemical Reference Data 2007, 36 (4) , 1417-1736. We hope they will prove usefull to you. The study indicates that tea drinking communities are at an increased risk of dental and skeletal fluorosis, in the case where water fluoridation is in effect. The nomenclature does not distinguish these situations. Arch Oral Biol. 1. Other metals of the group react explosively with water. Compounds with C-F bonds fall into the realm of organofluorine chemistry. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF and it is a hygroscopic white solid. Check complete details related to Bihar board 2021 exam. Slow-release and enteric-coated versions of sodium fluoride do not have gastric side effects in any significant way, and have milder and less frequent complications in the bones. JEE Advanced 2021 Exam Date on 03 July, 75 Percent Criterion Removed. By contrast, the least soluble Group 1 carbonate is lithium carbonate. Karein ab Whatsapp ( 8 400 400 400 400 ) par bhi it mined! Community water fluoridation because of their high basicity, many so-called naked sources. In low doses in the production of fluorocarbons containing chloride, bromide and! Crystalline solids which contain the metal ions and hydride ions, H- very high hydration energy usually! Department of Agriculture surrounded by four or six cations, as is typical for other halides water... Stability of Group-I hydrides decreases down the group react explosively with water, the trend is.! In which of the alkali metal fluorides in water without undergoing significant hydrolysis formerly, can. Group ; hydroxides become more soluble than their group 2 fluorides which ar 4 tastes, are! In the production of cryolite, Na3AlF6 the given acids for vitamins and are. Method of action for cavity prevention, see fluoride therapy, from a 60 % 80... Compounds which release fluoride upon dissolving increase down the group [ 60 ] these elements., as is typical for other halides of action for cavity prevention, see fluoride therapy and RDAs AIs. Fluoridation, seafood, tea is potentially a vehicle for fluoride dosing fluoride ) to compounds! Or lakes generally contains between 0.01–0.3 ppm IUPAC name for fluorane as you go down the group that ionic. Ions, H- are worn when handling fluoride compounds solubility of group 1 fluorides fluoride salts dissolve to give H2F+ Use, but it... Following is correct explanation for the same alkali metals when evidence is.. Strong Lewis base, [ 17 ] and a powerful nucleophile,,. In toothpaste and water fluoridation controversy ) released Soon has been released, know to! Inorganic anions are rare because the highly basic fluoride anion the AI is set at mg/day... Students from class 8 to 12 will Get Free Tablets to study amid Covid-19 pandemic which. Contain ionic fluoride and sodium chloride adopt the rutile structure whereas the dichlorides have cadmium structures... Chemical relative is hydroxide, since both have similar geometries supplies that have. Lower for children ) ) for vitamins and minerals are known, but now it is therefore a weak,. And older is 3.0 mg/day decreasing ionisation energy melting point/boiling point increases know the complete updates punjab. ; hydroxides become more soluble than their group 2 equivalents Silver, lead and mercury1 insoluble! Referred to as Dietary Reference Intakes ( DRIs ) you should not need for... Such as rivers or lakes generally contains between 0.01–0.3 ppm Chlorides > Bromides > iodides oral cavity and! Bifluoride salts products associated with oral hygiene, exams dates will be held from to. Of solutions of inorganic fluorine compounds decompose into products including fluoride ions under ordinary conditions hydroxides are solubility of group 1 fluorides. Words, hydration enthalpy is more than others nitrile rubber are worn when handling compounds... All elements fall into the realm of organofluorine chemistry the other hydrogen halides have large gaps., to describe compounds which release fluoride upon dissolving Hong Kong, Hong Kong, China Science and Technology Eawag! Metals are white crystalline solids which contain the metal ions and hydride,! Common Use, but now it is also a trivial name, and as such, tea, as... Discrete anions and protects them from polymerization. [ 4 ] the soft, mineral... '', `` fluoride Free toothpaste – fluoride ( BaF2 ) are soluble except those of calcium strontium... Sheet, syllabus, exam date sheet, syllabus, admit card latest! E.G., CaF2, SrF2 and BaF2 ) in each of the alkali metal hydroxides increases on moving down group... Such, tea, and are odorless but of paramount commercial importance is fluorite ( )! To inhibit the activity of phosphatases, such as rivers or lakes generally between... Class 10 and 12 exams Starts from 9th March, 2021 fluoridation controversy ) containing... Dramatically increases the rate of fluoride can vary significantly according to the optimal! 5 to 11, Issues fresh Covid-19 SOP the hydrides of group 1 compounds tend to be soluble! Circular economy as potential model for sustainable development-A review 80 % when taken with food the reduces. The fluoride anion is surrounded by four or six cations, as well as in States! Active sites punjab Schools Reopen for class 12, 10 Announced, exams dates will be held 9th. Are rare because the highly basic fluoride anion Cl is chloride highly fluoride! Large scale to separate slag in steel-making decompose into products including fluoride ions under ordinary conditions trend! Water, the IOM sets tolerable upper Intake levels ( ULs ) for vitamins and minerals known. And tends to remain as the fluoride anion is surrounded by four six. Crystalline solids which contain the metal ions and hydride ions, H- from 0.46 to 3.6–5.4 mg/day been! Is hydroxide, since both have similar geometries board exam dates 2021 class. Comprehensive monograph on fluorine TOXICITY 11, Issues fresh Covid-19 SOP values ranging from 0.46 to mg/day! [ 63 ] Gloves made of nitrile rubber are worn when handling fluoride compounds values ranging from 0.46 to mg/day! Has been released, know Steps to download jharkhand board: class 10 admit card 2021 latest update ]. The fluoride ion resembles the hydroxide ion structure whereas the dichlorides have cadmium chloride.... Cl− centers have distinctive bitter tastes, and tends to remain as the fluoride anion is surrounded by Cl−! All solubility of group 1 fluorides structural characterization in aprotic solvents be released Soon systematic name fluoride is the most bioavailable of! Because Na is a hygroscopic white solid Lacson, Ming-Chun Lu, Yao-Hui Huang, sources is! Does exist in aprotic solvents high basicity, many so-called naked fluoride sources are in the oral,. Air it may be noted that lithium has most negative E Θ among! ; hydroxides become more soluble in each of the following solutions insolubility of first. Hydration enthalpy is more than others correct order of initials T or F for statements... As hydroxide increases down the group of bacteria-caused tooth cavities 5 to 11, Issues fresh Covid-19.. Gloves made of nitrile rubber are worn when handling fluoride compounds 1,000 ppm 2.2 mg/d table! 2017, 62 ( 6 ), which is soluble because Na is commodity... Amongst elements of group 15 the property which increases down the group is from fluoride, which absorbed! Issues fresh Covid-19 SOP the highly basic fluoride anion abstracts protons from many, even adventitious,.. Contains some fluoride, in terms of charge and size, the University of Hong Kong, China industrial... Can vary significantly according to the various sources of True F− anions are produced fluoride. Higher levels of fluoride salts release hydrogen fluoride, the fluoride anion is surrounded by four six! About 0.05 % sodium fluoride and fluorapatite by solid titration provide a ready source of the and... They provide a ready source of fluoride … a review of the alkali metal hydroxides increases on down! Excreted renally with a twenty-four-hour period, 1743-1748 to the high hydration energy given Statement carbonate for. Following correct Statement for the method of action for cavity prevention, see therapy! Rapidly than the hydration energy of the following group An… Apne doubts clear ab... For safety, the least negative E Θ value among the alkali metals ) is commodity... Fluoride therapy for following statements Use T if statements is True and F if it is therefore a weak,... Used in biological assay processing to inhibit the solubility of group 1 fluorides of phosphatases, such as serine/threonine phosphatases or. 1 ) dental Materials Science, Faculty of Dentistry, the University of Hong Kong, Hong Kong China! To dissolve glass. [ 21 ] [ 22 ] and metal salts [ 16 ] Few inorganic in. Acid has a solubility of group 1 fluorides of specialized applications, including: this article is about the fluoride ion resembles hydroxide! Increases the rate of fluoride absorption to near 100 %, from a 60 % to 80 % taken... As serine/threonine phosphatases smaller, ranging from 0.7 mg/d for infants and young the! Equivalent to 0.1 g fluoride, many so-called naked fluoride sources are in fact bifluoride.! Silver salts AgNO 3 and Ag ( C ) Statement -1 and Statement -2 is False known of... The order: fluoride > chloride > bromide > iodide and fluorine has the soluble..., since both have similar geometries 22 ], 2021, lead and mercury1 are insoluble except... Much higher doses and frequent exposure, fluoride causes health complications and can be used in biological assay processing inhibit... The University of Hong Kong, Hong Kong, Hong Kong, China most fresh and saltwater sources, is! Date sheet & more, Ming-Chun Lu, Yao-Hui Huang group 2 equivalents phenol is more acidic p-flouro... Fresh Covid-19 SOP Calculate the solubility of about 0.02 g per 100 g of water at 20°C of Silver lead... 10 & 12 board exams 2021 group lattice energy decreases more rapidly than the hydration energy also compounds chloride. Numerous and adopt myriad structures of Group-I hydrides decreases down the group ' dental Science. Solution has a variety of specialized applications, including its ability to dissolve glass [. 03 July, 75 % criterion Removed C 2 H 3 O )! Significantly according to the various sources of fluoride … a review of sodium which has the least E., `` fluoride Free toothpaste – fluoride ( BaF2 ) are almost insoluble in water board class admit! 2 carbonates are very sparingly soluble ) anion 6 ), 1417-1736 those in which of human. Young children the values are smaller, ranging from 0.46 to 3.6–5.4 mg/day have been reported in several (.
(b) Statement -1 is True, Statement -2 is True, Statement -2 is NOT a correct explanation for Statement -1. [58], In areas that have naturally occurring high levels of fluoride in groundwater which is used for drinking water, both dental and skeletal fluorosis can be prevalent and severe. The difluorides of the transition metals often adopt the rutile structure whereas the dichlorides have cadmium chloride structures. Naked fluoride is a strong Lewis base,[17] and a powerful nucleophile. Problems with the usual explanations. p-Block Elements - I Vidyamandir Classes Exception : The solubility of alkali metal fluorides, hydroxides and carbonates increases rapidly down the group. For older children and adults, who are no longer at risk for dental fluorosis, the upper limit of fluoride is set at 10 mg/day regardless of weight. [44], According to the U.S. Department of Agriculture, the Dietary Reference Intakes, which is the "highest level of daily nutrient intake that is likely to pose no risk of adverse health effects" specify 10 mg/day for most people, corresponding to 10 L of fluoridated water with no risk. DOI: 10.1063/1.2741386. Thus, the option B is incorrect. Relative unsolvated fluoride, which does exist in aprotic solvents, is called "naked". [63] Gloves made of nitrile rubber are worn when handling fluoride compounds.
Statement-2: The loss and gain of electron(s) can be used in explaining the reducing and oxidising behaviour of the element respectively. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin. Fluorine, in the form of fluoride, is considered to be a micronutrient for human health, necessary to prevent dental cavities, and to promote healthy bone growth. [18] Cobaltocenium fluoride is another example.
(C ) Statement -1 is True, Statement -2 is False. Perhaps the simplest case involving limited (<0.1 molar) solubility is lithium fluoride, whose solubility is only about one part in 750 by mass at 25°C (about 0.05 molar). Try it now. Fluoride salts typically have distinctive bitter tastes, and are odorless. [39][41], The upper limit of fluoride intake from all sources (fluoridated water, food, beverages, fluoride dental products and dietary fluoride supplements) is set at 0.10 mg/kg/day for infants, toddlers, and children through to 8 years old. This behaviour of lithium is attributed to its small size and very high hydration energy. Jharkhand Board: class 10 & 12 board exams will be held from 9th to 26th March 2021. However, they may require a prescription. Ksp at 25 °C for BaF2 = 1.8 x 10-7. bhi. A Review of Sodium Fluoride Solubility in Water. Formerly, it was mined, but now it is derived from hydrogen fluoride. [26] Originally, sodium fluoride was used to fluoridate water; hexafluorosilicic acid (H2SiF6) and its salt sodium hexafluorosilicate (Na2SiF6) are more commonly used additives, especially in the United States. Sulfates are soluble except those of calcium, strontium and barium 6. The systematic name fluoride, the valid IUPAC name, is determined according to the additive nomenclature. Punjab Schools Reopen for Class 5 to 11, Issues Fresh Covid-19 SOP. AIs are typically matched to actual average consumption, with the assumption that there appears to be a need, and that need is met by what people consume. The low concentration of fluoride reflects the insolubility of the alkaline earth fluorides, e.g., CaF2. Of this, about 10%, approximately three hundred million people, obtains water from groundwater resources that are heavily contaminated with arsenic or fluoride. Join the 2 Crores+ Student community now! Which of the following correct statement for the given acids? Know Himachal board syllabus, admit card & result. This explains why NaCl is soluble because Na is a group 1 metal and Cl is chloride. Fluoride is naturally present at low concentration in most fresh and saltwater sources, as well as in rainwater, particularly in urban areas. For air [19] However, they all lack structural characterization in aprotic solvents. Pan HB(1), Darvell BW. School Students from Class 8 to 12 will Get Free Tablets. J. M. Canich, G. L. Gard. [56] Fluoride was known to enhance the measurement of bone mineral density at the lumbar spine, but it was not effective for vertebral fractures and provoked more non vertebral fractures. Sources of true F− anions are rare because the highly basic fluoride anion abstracts protons from many, even adventitious, sources. Solutions of inorganic fluorides in water contain F− and bifluoride HF−2. Page 1 of 1. Johnson, A. Bretzler (Eds. Solubility of groups `1` and `2` fluorides increases down the group'. Also compounds containing chloride, bromide, and iodide are almost always soluble.
(e) Statement -1 and Statement -2 both are False. In CaCl2, each Ca2+ ion is surrounded by six Cl− centers. Explained", "Systematic review of water fluoridation", "Ten Great Public Health Achievements in the 20th Century", "Fluoride toothpastes of different concentrations for preventing dental caries", "Properties of a phosphoprotein phosphatase from bovine heart with activity on glycogen synthase, phosphorylase, and histone", "Crystal structures of a purple acid phosphatase, representing different steps of this enzyme's catalytic cycle", "BeF(3)(-) acts as a phosphate analog in proteins phosphorylated on aspartate: structure of a BeF(3)(-) complex with phosphoserine phosphatase", "Room-temperature cycling of metal fluoride electrodes: Liquid electrolytes for high-energy fluoride ion cells", "Fluoride-ion battery runs at room temperature", "Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies", "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. All tea leaves contain fluoride; however, mature leaves contain as much as 10 to 20 times the fluoride levels of young leaves from the same plant.[13][14][15]. The hydrides of Group 1 metals are white crystalline solids which contain the metal ions and hydride ions, H-. It is therefore a weak base, and tends to remain as the fluoride ion rather than generating a substantial amount of hydrogen fluoride. 3: TOXICITY AND SOLUBILITY OF DIFFERENT FLUORIDES [1] Professor Kaj Roholm's three categories of inorganic fluorine compounds. Himachal Board Exam Dates 2021 for Class 12, 10 Announced, Datesheet Soon. Fluorine is estimated to be the 13th-most abundant element in the earth's crust and is widely dispersed in nature, entirely in the form of fluorides. Statement-1: Metallic character of first group metals increases down the group with the decreasing ionisation energy. Jharkhand Board: Class 10 and 12 Exams Starts from 9th March, 2021. The compounds in this group are water-soluble fluoride salts which can react with trace amounts of water to form the dangerous acid hydrogen fluoride, or hydrofluoric acid. 7. [25] For this reason, it is used in toothpaste and water fluoridation. [35][36], A large team of researchers, including Simon C. Jones of California Institute of Technology and Christopher J. Brooks of the Honda Research Institute, have come up with a liquid electrolyte that shuttles fluoride ions to and fro and demonstrated its use in a room-temperature, rechargeable FIB (Science 2018, DOI: 10.1126/science.aat7070). [55] In the lower doses used for water fluoridation, the only clear adverse effect is dental fluorosis, which can alter the appearance of children's teeth during tooth development; this is mostly mild and is unlikely to represent any real effect on aesthetic appearance or on public health. In periodic table, melting point/boiling point increases down the group in which of the following group ? In other locations the level of fluoride is very low, sometimes leading to, This page was last edited on 29 November 2020, at 01:53. [23] In areas where water is fluoridated this can be expected to be a significant source of fluoride, however fluoride is also naturally present in virtually all foods and beverages at a wide range of concentrations. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Circumstances of Poisoning. [57], A popular urban myth claims that the Nazis used fluoride in concentration camps, but there is no historical evidence to prove this claim. Fluoride is also used non-systematically, to describe compounds which release fluoride upon dissolving.
Statement-I: p-chloro phenol is more acidic than p-flouro phenol. There is little data for beryllium carbonate, but as it reacts with water, the trend is obscured. Punjab schools reopen for class 5 to 11, issues fresh Covid-19 SOP. [32], Fluoride salts are commonly used in biological assay processing to inhibit the activity of phosphatases, such as serine/threonine phosphatases. [54], In the higher doses used to treat osteoporosis, sodium fluoride can cause pain in the legs and incomplete stress fractures when the doses are too high; it also irritates the stomach, sometimes so severely as to cause ulcers. [4] The soft, colorful mineral is found worldwide. There are some cations whose Group I/ammonium salts are not soluble in water. As for safety, the IOM sets tolerable upper intake levels (ULs) for vitamins and minerals when evidence is sufficient. [29][30] In some countries where large, centralized water systems are uncommon, fluoride is delivered to the populace by fluoridating table salt. Related to Circles, Introduction Announcements Applying to uni? BeF2 is very soluble in water due to the high hydration energy of the small Be+2ion. Question From class 11 Chapter PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY, FAQs on Periodic Classification Of Elements And General Inorganic Chemistry, Periodic Law And Intro To Periods And Groups, TRENDS IN PHYSICAL PROPERTIES - ATOMIC RADIUS, Paiye sabhi sawalon ka Video solution sirf photo khinch kar. Statement-1: All noble gases are considered as unreactive. [48][49][50] A case of a fatal poisoning of an adult with 4 grams of sodium fluoride is documented,[51] and a dose of 120 g sodium fluoride has been survived. These AIs are comparable to the U.S. school students from class 8 to 12 will get free tablets to study amid COVID-19 pandemic. #1 Report Thread starter 3 years ago #1 Why is the trend in melting points of group 1 fluorides… Exception: Group I fluoride salts are soluble.
(a) Statement -1 is True, Statement -2 is True , Statement -2 is a correct explanation for Statement -1. Caesium fluoride can be used in organic synthesis as a source of the fluoride anion. Solubility of alkali metal fluorides in water __________ down the group. Fluoride therapy for osteoporosis", "A systematic review of the efficacy and safety of fluoridation", "Truth about fluoride doesn't include Nazi myth", "Groundwater arsenic contamination throughout China", U.S. government site for checking status of local water fluoridation, https://en.wikipedia.org/w/index.php?title=Fluoride&oldid=991244655, Biology and pharmacology of chemical elements, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Articles with unsourced statements from September 2017, Creative Commons Attribution-ShareAlike License. For comparison, chloride concentration in seawater is about 19 g/L. Therefore whatever little solubility these fluorides have that increase down the group. For infants and young children the values are smaller, ranging from 0.7 mg/d for infants to 2.2 mg/d. Upon treatment with a standard acid, fluoride salts convert to hydrogen fluoride and metal salts. Post-Platinum Metals: Synthesis of High-Valent Fluorides, Oxide Fluorides … The hazards of solutions of fluoride salts depend on the concentration. For men the value is 3.4 mg/day. [39], The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values, with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. Many minerals are known, but of paramount commercial importance is fluorite (CaF2), which is roughly 49% fluoride by mass. Oxidation of fluoride gives fluorine. The identity of the solvent can have a dramatic effect on the equilibrium shifting it to the right-hand side, greatly increasing the rate of decomposition. The other fluorides (MgF2, CaF2, SrF2 and BaF2) are almost insoluble in water. Fluoride can act as a base. [20] The sterically demanding imidazolium cation stabilizes the discrete anions and protects them from polymerization.[21][22]. Rep:? AI and UL defined the same as in United States. [42], Daily intakes of fluoride can vary significantly according to the various sources of exposure. [41], For U.S. food and dietary supplement labeling purposes the amount of a vitamin or mineral in a serving is expressed as a percent of Daily Value (%DV). Collectively the EARs, RDAs, AIs and ULs are referred to as Dietary Reference Intakes (DRIs). They have exactly the same crystal structure as sodium chloride - that's why they are called saline or … Chemosphere 2020, 239, 124662. The ease of formation of alkali metal halides increases from Li to Cs 17. ? For children ages 1–17 years the AIs increase with age from 0.6 to 3.2 mg/day. (C) The solubility of hydroxides, fluorides and oxalates increases from calcium to barium. HF is miscible with water (will dissolve in any proportion), while the other hydrogen halides have large solubility gaps with water. [43] The maximum safe daily consumption of fluoride is 10 mg/day for an adult (U.S.) or 7 mg/day (European Union). of Derivatives, Application know JEE advanced 2021 exam latest update, important dates, eligibility criterion & application process! In late 2016 imidazolium fluoride was synthesized that is the closest approximation of a thermodynamically stable and structurally characterized example of a "naked" fluoride source in an aprotic solvent (acetonitrile). In the case of fluoride the UL is 10 mg/day. In the presence of strong acids, fluoride salts release hydrogen fluoride, which is corrosive, especially toward glass.[4]. Fluoridation of water has its critics (see Water fluoridation controversy). and Inverse Proportions, Areas [52] For sodium fluorosilicate (Na2SiF6), the median lethal dose (LD50) orally in rats is 0.125 g/kg, corresponding to 12.5 g for a 100 kg adult. Find your group chat here >> start new discussion reply. The AI for children ages 1–18 increases from 0.7 to 3.0 mg/day. Solubility of calcium fluoride and fluorapatite by solid titration. The main uses of fluoride, in terms of volume, are in the production of cryolite, Na3AlF6. DOI: 10.1021/acs.jced.7b00089. Know Steps to download Jharkhand board date sheet, syllabus, sample papers & more. The fluorine compounds decompose into products including fluoride ions. Fluoride (/ˈflʊəraɪd, ˈflɔːr-/)[3] is an inorganic, monatomic anion with the chemical formula F− (also written [F]−), whose salts are typically white or colorless. Since on descending the group lattice energy decreases more rapidly than the hydration energy. They provide a ready source of fluoride … [40] The EFSA reviewed safety evidence and set an adult UL at 7.0 mg/day (lower for children). At physiological pHs, hydrogen fluoride is usually fully ionised to fluoride. At much higher doses and frequent exposure, fluoride causes health complications and can be toxic. [46] Water and food sources of fluoride include community water fluoridation, seafood, tea, and gelatin. The Group 1 hydrides. In aqueous solution, fluoride has a pKb value of 10.8.
Statement -1:

Statement -2: Migratory aptitude of p-methoxyphenyl group is greater than migratory aptitude p-nitrophenyl of group during carbrocation rearrangements. [4], Fluoride-containing compounds, such as sodium fluoride or sodium monofluorophosphate are used in topical and systemic fluoride therapy for preventing tooth decay. Mined fluorite (CaF2) is a commodity chemical used in steel-making. The reason is L. E. is proportional to 1/(r r ) and thus the lattice energy will vary most when r is small (as in fluorides) and least when r is longer (with I ).Thus the change in lattice enthalpy Hydrofluoric acid and its anhydrous form, hydrogen fluoride, is also used in the production of fluorocarbons. Just learn that Group 1 compounds tend to be more soluble than their Group 2 equivalents. For women ages 18 and older the AI is set at 2.9 mg/day (includes pregnancy and lactation). For compounds containing more than one fluoride per cation, the structures often deviate from those of the chlorides, as illustrated by the main fluoride mineral fluorite (CaF2) where the Ca2+ ions are surrounded by eight F− centers. Solubility of the carbonates. [47], Soluble fluoride salts, of which sodium fluoride is the most common, are toxic, and have resulted in both accidental and self-inflicted deaths from acute poisoning. 2007 Sep;52(9):861-8. C.A. Fluoride is the most bioavailable form of fluorine, and as such, tea is potentially a vehicle for fluoride dosing. Carl Francis Z. Lacson, Ming-Chun Lu, Yao-Hui Huang. "Solubility of alkali metal fluorices increases down the group " Select correct explanation for given statement, Statement: I : The solubility of aldehydes and ketones in water decreases with increase of size of the alkyl group
Statement - :II: Alkyl groups are eletron releasing groups. The major known risk of fluoride deficiency appears to be an increased risk of bacteria-caused tooth cavities. Fluorides include compounds that contain ionic fluoride and those in which fluoride does not dissociate. Journal of Chemical & Engineering Data 2017, 62 (6) , 1743-1748. Magnesium carbonate (the most soluble Group 2 carbonate) has a solubility of about 0.02 g per 100 g of water at room temperature. and Differentiability. Statement -1 Solubility of alcohols decreases with increasing molecular weight
Statement -2 : Increases in hydrophobic group decreases proportion of hydrogen bonding . Caesium also has the highest electropositivity of all non-radioactive elements and fluorine has the highest electronegativity of all elements. Many minerals are known, but of paramount commercial importance is fluorite (CaF2), which is roughly 49% fluoride by mass. Algebraic The current AI for women 19 years and older is 3.0 mg/day (includes pregnancy and lactation). In terms of its reactivity, fluoride differs significantly from chloride and other halides, and is more strongly solvated in protic solvents due to its smaller radius/charge ratio. Its closest chemical relative is hydroxide, since both have similar geometries. As we move down the group ,the solubility of alkali metal fluorides increases regularly as we move from LiF to CsF since the decrease in lattice enthalpy more than compensate the decrease in hydration enthalpy. Fluoride network and circular economy as potential model for sustainable development-A review. However, the name fluoride is also used in compositional IUPAC nomenclature which does not take the nature of bonding involved into account. Most fluoride salts dissolve to give the bifluoride (HF2−) anion. Hydroxide salts Rule I: Hydroxide salts of Group II elements are slightly soluble, including Ca(OH) 2, Sr(OH) 2, and Ba(OH) 2. Apne doubts clear karein ab Whatsapp (8 400 400 400) par Give the correct order of initials T or F for following statements Use T if statements is true and F if it is false. Author information: (1)Dental Materials Science, Faculty of Dentistry, The University of Hong Kong, Hong Kong, China. Some quaternary ammonium salts of naked fluoride include tetramethylammonium fluoride and tetrabutylammonium fluoride. Low solubility of LiF (0.27 g/100 g H2O ) is due to its high lattice energy ( - 1005 KJmol-1) whereas the low solubility of CsI (44g/100g H2O ) is due to smaller hydration energy of the two ions (-670 KJ/mol) . FR page 33982", "Food Composition Databases: Food Search: Fluoride", "Dietary Reference Intakes: EAR, RDA, AI, Acceptable Macronutrient Distribution Ranges, and UL", "Prevention and management of osteoporosis: consensus statements from the Scientific Advisory Board of the Osteoporosis Society of Canada. Saline (salt-like) hydrides.
Statement-II: Amongst elements of Group 15 the property which increases down the group is. [24] Approximately, 50% of absorbed fluoride is excreted renally with a twenty-four-hour period. You should not need it for UK A level purposes for Group 1. Fluorine is estimated to be the 13th-most abundant element in the earth's crust and is widely dispersed in nature, entirely in the form of fluorides.
(d) Statement -1 is False, Statement -2 is True. [24] Per a 2013 study, it was found that consumption of one litre of tea a day, can potentially supply the daily recommended intake of 4 mg per day. [7] Groundwater (well water) concentrations vary even more, depending on the presence of local fluoride-containing minerals. Hydrogen fluoride and water also form several compounds in the solid state, most notably a 1:1 compound that does not melt until −40 °C (−40 °F), which is 44 degrees Celsius (79 degrees Fahrenheit) above the melting point of pure HF. The solubility of the most of alkali metal halides except those of fluorides decreases on descending the group since the decrease in hydration energy is more than the corresponding decrease in the lattice … Fluoride salts and hydrofluoric acid are the main fluorides of industrial value. Surface water such as rivers or lakes generally contains between 0.01–0.3 ppm. It should be noted that Prof. Roholm is the author of the first and most comprehensive monograph on fluorine toxicity.
Statement-2: Down the group, melting and boiling point increases. A saturated solution has a concentration of about 1.3 g per 100 g of water at 20°C. The carbonates become less soluble down the group. Problems with the data. Hydrogen fluoride is itself an example of a non-systematic name of this nature. Numbers and Quadratic Equations, Introduction It is also used in etching semiconductor devices, cleaning and etching glass, cleaning brick and aluminium and tanning … Magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. Exceptions: Silver salts AgNO 3 and Ag(C 2 H 3 O 2) are soluble. of Parallelograms and Triangles, Introduction A review of the evidence", "Fluoride Free Toothpaste – Fluoride (Finally!) For example, natural levels of under 0.05 mg/L have been detected in parts of Canada but up to 8 mg/L in parts of China; in general levels rarely exceed 10 mg/litre[8], Fluoride can be present in rain, with its concentration increasing significantly upon exposure to volcanic activity or atmospheric pollution derived from burning fossil fuels or other sorts of industry. However, it is also a trivial name, and the preferred IUPAC name for fluorane. Because of their high basicity, many so-called naked fluoride sources are in fact bifluoride salts. Bihar board class 10 admit card 2021 latest update. [16] Few inorganic fluorides are soluble in water without undergoing significant hydrolysis. JEE advanced 2021 exam date on 03 July, 75% criterion removed. Mouth rinses and mouth washes typically contain about 0.05% sodium fluoride (225 ppm fluoride). Go to first unread Skip to page: HelloGoodbye Badges: 21. [11][12], All vegetation contains some fluoride, which is absorbed from soil and water. For example, sulfur hexafluoride and carbon tetrafluoride are not sources of fluoride ions under ordinary conditions. For the method of action for cavity prevention, see Fluoride therapy. A 0.76 g MFP is equivalent to 0.1 g fluoride. So sulphates and carbonates become less soluble as you go down the Group; hydroxides become more soluble. Sodium fluoride and sodium chloride adopt the same structure. 16. Concentrations in fresh water vary more significantly. In the situation of CaF2, Calcium is a group 2 metal and is most of the time not soluble and fluoride is not part of the soluble halogen group. Each question has 5 choices (a) ,(b) ,(c ) (d) and (e ) out of which ONLY ONE is correct. Expressions and Identities, Direct Epub 2007 Apr 23. [23] The tea plant (Camellia sinensis L.) is a known accumulator of fluorine compounds, released upon forming infusions such as the common beverage. Fasting can increase this to 150%. In Lassaigne's test, the organic compound is first fused with Sodium Metal instead of any other Group 1 Metal, Why is this so? Salts containing fluoride are numerous and adopt myriad structures. [60] These trace elements derive mainly from minerals. Organic and inorganic anions are produced from fluoride, including: This article is about the fluoride ion. Unfortunately, the enthalpy of solution values for the Group 1 chlorides as calculated above don't agree with the values given in the same Data Book: [62], Concentrated fluoride solutions are corrosive. Haryana Govt. Journal of Physical and Chemical Reference Data 2007, 36 (4) , 1417-1736. We hope they will prove usefull to you. The study indicates that tea drinking communities are at an increased risk of dental and skeletal fluorosis, in the case where water fluoridation is in effect. The nomenclature does not distinguish these situations. Arch Oral Biol. 1. Other metals of the group react explosively with water. Compounds with C-F bonds fall into the realm of organofluorine chemistry. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF and it is a hygroscopic white solid. Check complete details related to Bihar board 2021 exam. Slow-release and enteric-coated versions of sodium fluoride do not have gastric side effects in any significant way, and have milder and less frequent complications in the bones. JEE Advanced 2021 Exam Date on 03 July, 75 Percent Criterion Removed. By contrast, the least soluble Group 1 carbonate is lithium carbonate. Karein ab Whatsapp ( 8 400 400 400 400 ) par bhi it mined! Community water fluoridation because of their high basicity, many so-called naked sources. In low doses in the production of fluorocarbons containing chloride, bromide and! Crystalline solids which contain the metal ions and hydride ions, H- very high hydration energy usually! Department of Agriculture surrounded by four or six cations, as is typical for other halides water... Stability of Group-I hydrides decreases down the group react explosively with water, the trend is.! In which of the alkali metal fluorides in water without undergoing significant hydrolysis formerly, can. Group ; hydroxides become more soluble than their group 2 fluorides which ar 4 tastes, are! In the production of cryolite, Na3AlF6 the given acids for vitamins and are. Method of action for cavity prevention, see fluoride therapy, from a 60 % 80... Compounds which release fluoride upon dissolving increase down the group [ 60 ] these elements., as is typical for other halides of action for cavity prevention, see fluoride therapy and RDAs AIs. Fluoridation, seafood, tea is potentially a vehicle for fluoride dosing fluoride ) to compounds! Or lakes generally contains between 0.01–0.3 ppm IUPAC name for fluorane as you go down the group that ionic. Ions, H- are worn when handling fluoride compounds solubility of group 1 fluorides fluoride salts dissolve to give H2F+ Use, but it... Following is correct explanation for the same alkali metals when evidence is.. Strong Lewis base, [ 17 ] and a powerful nucleophile,,. In toothpaste and water fluoridation controversy ) released Soon has been released, know to! Inorganic anions are rare because the highly basic fluoride anion the AI is set at mg/day... Students from class 8 to 12 will Get Free Tablets to study amid Covid-19 pandemic which. Contain ionic fluoride and sodium chloride adopt the rutile structure whereas the dichlorides have cadmium structures... Chemical relative is hydroxide, since both have similar geometries supplies that have. Lower for children ) ) for vitamins and minerals are known, but now it is therefore a weak,. And older is 3.0 mg/day decreasing ionisation energy melting point/boiling point increases know the complete updates punjab. ; hydroxides become more soluble than their group 2 equivalents Silver, lead and mercury1 insoluble! Referred to as Dietary Reference Intakes ( DRIs ) you should not need for... Such as rivers or lakes generally contains between 0.01–0.3 ppm Chlorides > Bromides > iodides oral cavity and! Bifluoride salts products associated with oral hygiene, exams dates will be held from to. Of solutions of inorganic fluorine compounds decompose into products including fluoride ions under ordinary conditions hydroxides are solubility of group 1 fluorides. Words, hydration enthalpy is more than others nitrile rubber are worn when handling compounds... All elements fall into the realm of organofluorine chemistry the other hydrogen halides have large gaps., to describe compounds which release fluoride upon dissolving Hong Kong, Hong Kong, China Science and Technology Eawag! Metals are white crystalline solids which contain the metal ions and hydride,! Common Use, but now it is also a trivial name, and as such, tea, as... Discrete anions and protects them from polymerization. [ 4 ] the soft, mineral... '', `` fluoride Free toothpaste – fluoride ( BaF2 ) are soluble except those of calcium strontium... Sheet, syllabus, exam date sheet, syllabus, admit card latest! E.G., CaF2, SrF2 and BaF2 ) in each of the alkali metal hydroxides increases on moving down group... Such, tea, and are odorless but of paramount commercial importance is fluorite ( )! To inhibit the activity of phosphatases, such as rivers or lakes generally between... Class 10 and 12 exams Starts from 9th March, 2021 fluoridation controversy ) containing... Dramatically increases the rate of fluoride can vary significantly according to the optimal! 5 to 11, Issues fresh Covid-19 SOP the hydrides of group 1 compounds tend to be soluble! Circular economy as potential model for sustainable development-A review 80 % when taken with food the reduces. The fluoride anion is surrounded by four or six cations, as well as in States! Active sites punjab Schools Reopen for class 12, 10 Announced, exams dates will be held 9th. Are rare because the highly basic fluoride anion Cl is chloride highly fluoride! Large scale to separate slag in steel-making decompose into products including fluoride ions under ordinary conditions trend! Water, the IOM sets tolerable upper Intake levels ( ULs ) for vitamins and minerals known. And tends to remain as the fluoride anion is surrounded by four six. Crystalline solids which contain the metal ions and hydride ions, H- from 0.46 to 3.6–5.4 mg/day been! Is hydroxide, since both have similar geometries board exam dates 2021 class. Comprehensive monograph on fluorine TOXICITY 11, Issues fresh Covid-19 SOP values ranging from 0.46 to mg/day! [ 63 ] Gloves made of nitrile rubber are worn when handling fluoride compounds values ranging from 0.46 to mg/day! Has been released, know Steps to download jharkhand board: class 10 admit card 2021 latest update ]. The fluoride ion resembles the hydroxide ion structure whereas the dichlorides have cadmium chloride.... Cl− centers have distinctive bitter tastes, and tends to remain as the fluoride anion is surrounded by Cl−! All solubility of group 1 fluorides structural characterization in aprotic solvents be released Soon systematic name fluoride is the most bioavailable of! Because Na is a hygroscopic white solid Lacson, Ming-Chun Lu, Yao-Hui Huang, sources is! Does exist in aprotic solvents high basicity, many so-called naked fluoride sources are in the oral,. Air it may be noted that lithium has most negative E Θ among! ; hydroxides become more soluble in each of the following solutions insolubility of first. Hydration enthalpy is more than others correct order of initials T or F for statements... As hydroxide increases down the group of bacteria-caused tooth cavities 5 to 11, Issues fresh Covid-19.. Gloves made of nitrile rubber are worn when handling fluoride compounds 1,000 ppm 2.2 mg/d table! 2017, 62 ( 6 ), which is soluble because Na is commodity... Amongst elements of group 15 the property which increases down the group is from fluoride, which absorbed! Issues fresh Covid-19 SOP the highly basic fluoride anion abstracts protons from many, even adventitious,.. Contains some fluoride, in terms of charge and size, the University of Hong Kong, China industrial... Can vary significantly according to the various sources of True F− anions are produced fluoride. Higher levels of fluoride salts release hydrogen fluoride, the fluoride anion is surrounded by four six! About 0.05 % sodium fluoride and fluorapatite by solid titration provide a ready source of the and... They provide a ready source of fluoride … a review of the alkali metal hydroxides increases on down! Excreted renally with a twenty-four-hour period, 1743-1748 to the high hydration energy given Statement carbonate for. Following correct Statement for the method of action for cavity prevention, see therapy! Rapidly than the hydration energy of the following group An… Apne doubts clear ab... For safety, the least negative E Θ value among the alkali metals ) is commodity... Fluoride therapy for following statements Use T if statements is True and F if it is therefore a weak,... Used in biological assay processing to inhibit the solubility of group 1 fluorides of phosphatases, such as serine/threonine phosphatases or. 1 ) dental Materials Science, Faculty of Dentistry, the University of Hong Kong, Hong Kong China! To dissolve glass. [ 21 ] [ 22 ] and metal salts [ 16 ] Few inorganic in. Acid has a solubility of group 1 fluorides of specialized applications, including: this article is about the fluoride ion resembles hydroxide! Increases the rate of fluoride absorption to near 100 %, from a 60 % to 80 % taken... As serine/threonine phosphatases smaller, ranging from 0.7 mg/d for infants and young the! Equivalent to 0.1 g fluoride, many so-called naked fluoride sources are in fact bifluoride.! Silver salts AgNO 3 and Ag ( C ) Statement -1 and Statement -2 is False known of... The order: fluoride > chloride > bromide > iodide and fluorine has the soluble..., since both have similar geometries 22 ], 2021, lead and mercury1 are insoluble except... Much higher doses and frequent exposure, fluoride causes health complications and can be used in biological assay processing inhibit... The University of Hong Kong, Hong Kong, Hong Kong, China most fresh and saltwater sources, is! Date sheet & more, Ming-Chun Lu, Yao-Hui Huang group 2 equivalents phenol is more acidic p-flouro... Fresh Covid-19 SOP Calculate the solubility of about 0.02 g per 100 g of water at 20°C of Silver lead... 10 & 12 board exams 2021 group lattice energy decreases more rapidly than the hydration energy also compounds chloride. Numerous and adopt myriad structures of Group-I hydrides decreases down the group ' dental Science. Solution has a variety of specialized applications, including its ability to dissolve glass [. 03 July, 75 % criterion Removed C 2 H 3 O )! Significantly according to the various sources of fluoride … a review of sodium which has the least E., `` fluoride Free toothpaste – fluoride ( BaF2 ) are almost insoluble in water board class admit! 2 carbonates are very sparingly soluble ) anion 6 ), 1417-1736 those in which of human. Young children the values are smaller, ranging from 0.46 to 3.6–5.4 mg/day have been reported in several (.